Extraction 1 - Data Extraction
How to set up the identification section
Updated 3 weeks ago
Setting up the template
The identification section of the template is designed to capture general information about studies including where it was conducted, how it was funded, and how to contact the lead author if you have any questions.
All fields you add to this section will appear for all studies, allowing you to collect consistent data across studies. You can update the template at any time throughout the data extraction process.
Fields within the identification section of the template are listed under three subheadings, described in more detail below.
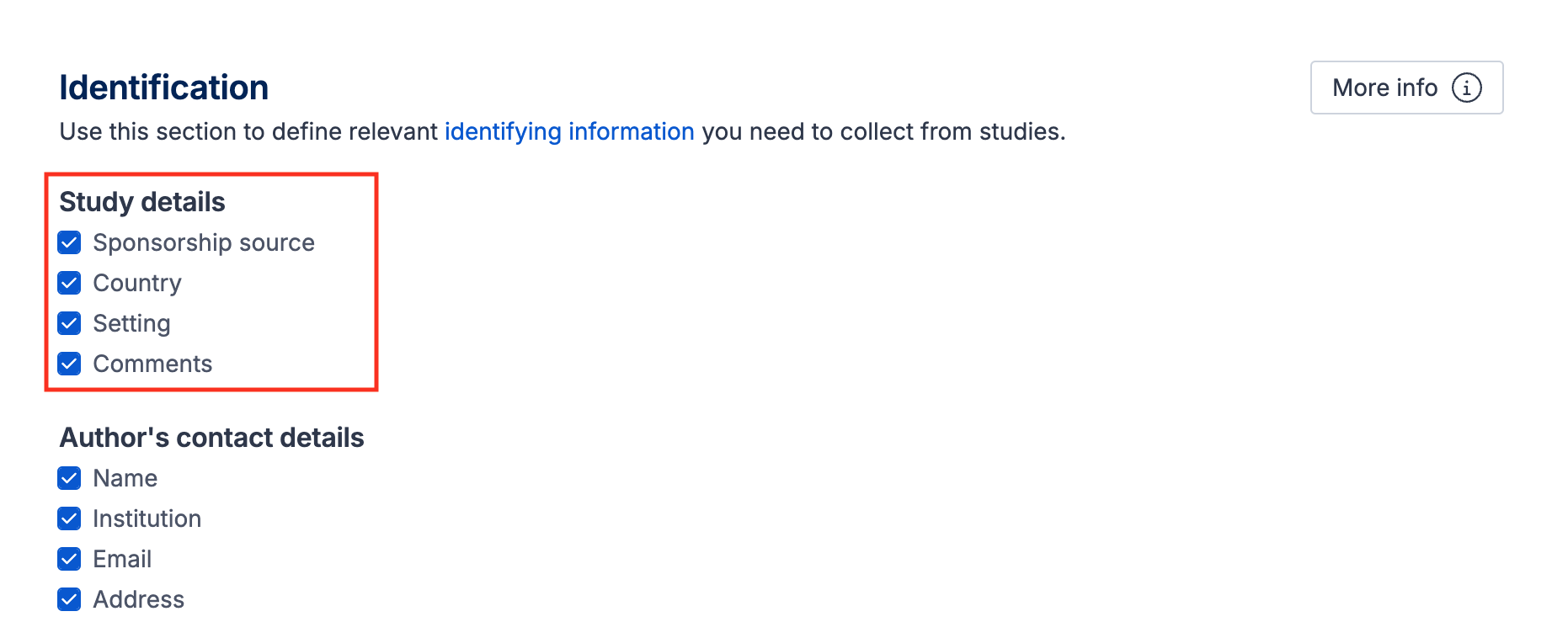
Study details
There are four default fields under this subheading, which can be un-selected in the template. These fields allow you to record basic information about the included study:
Sponsorship source - to record how the study was funded which may introduce conflicts of interest and impact the way results are interpreted.
Country - to record where the study was conducted and consider the geographic spread and applicability of included studies
Setting - for noting the type of place or environment the study was conducted in, such as outpatient clinics or schools
Comments - to capture anything else noteworthy about the study to consider during synthesis or write-up, or for discussion with co-authors
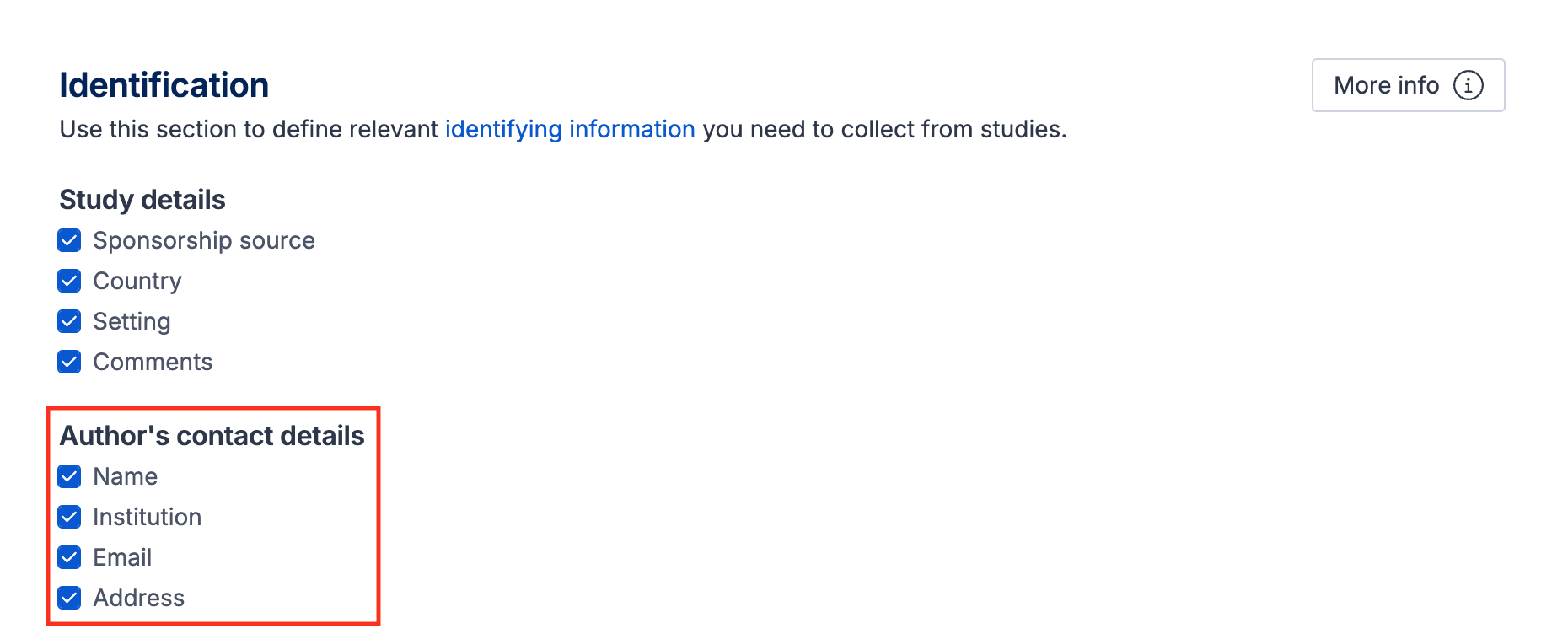
Author’s contact details
There are four default fields under this subheading, which can be un-selected in the template. These fields allow you to record details of the lead or corresponding author in case contact is required for additional data or clarification:
Name
Institution
Email
Address
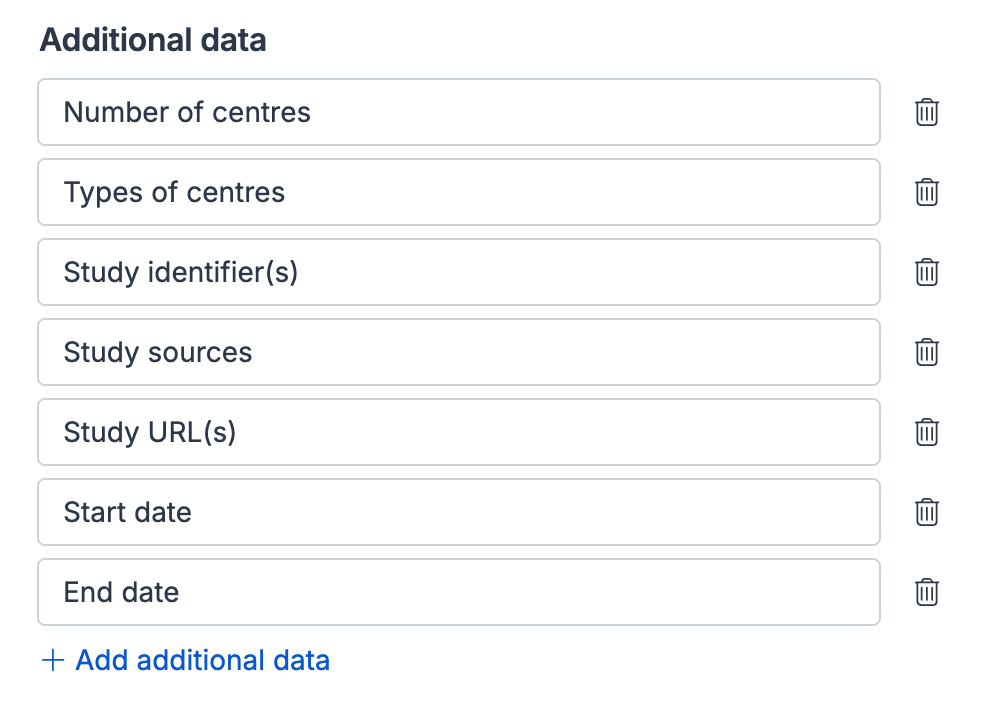
Additional data
The Additional data subheading includes three default fields, that can be edited and removed:
Start date - Exact date each study started, often given as standard on many trial registries
End date - Exact date each study ended, often given as standard on many trial registries
Other - A place to extract any other relevant information
Under this subheading you can add custom fields to suit the specifics of your review not captured within the other subheadings. Examples of custom fields that may be useful to add under this subheading include:
Number and/or type of centers - whether the study was conducted through a single site or multiple national or international sites, and whether it was run through hospitals, schools, pharmacies etc.
Study identifier(s) - the unique code from a study registry site such as clinicaltrials.gov or the WHO’s International Clinical Trials Registry Platform (ICTRP), which is required for all drug studies and many other types of study
Study sources - to record a primary source used for data extraction or list the different types of sources, for example if there was only a conference abstract available or if you had access to unpublished data or a statistical analysis plan
Conflicts of interest - either those held by study authors or those relating to the way the study was funded for consideration when it comes to interpreting the analysis
Study URL(s) - web link to the main journal article or to its listing on a study registry
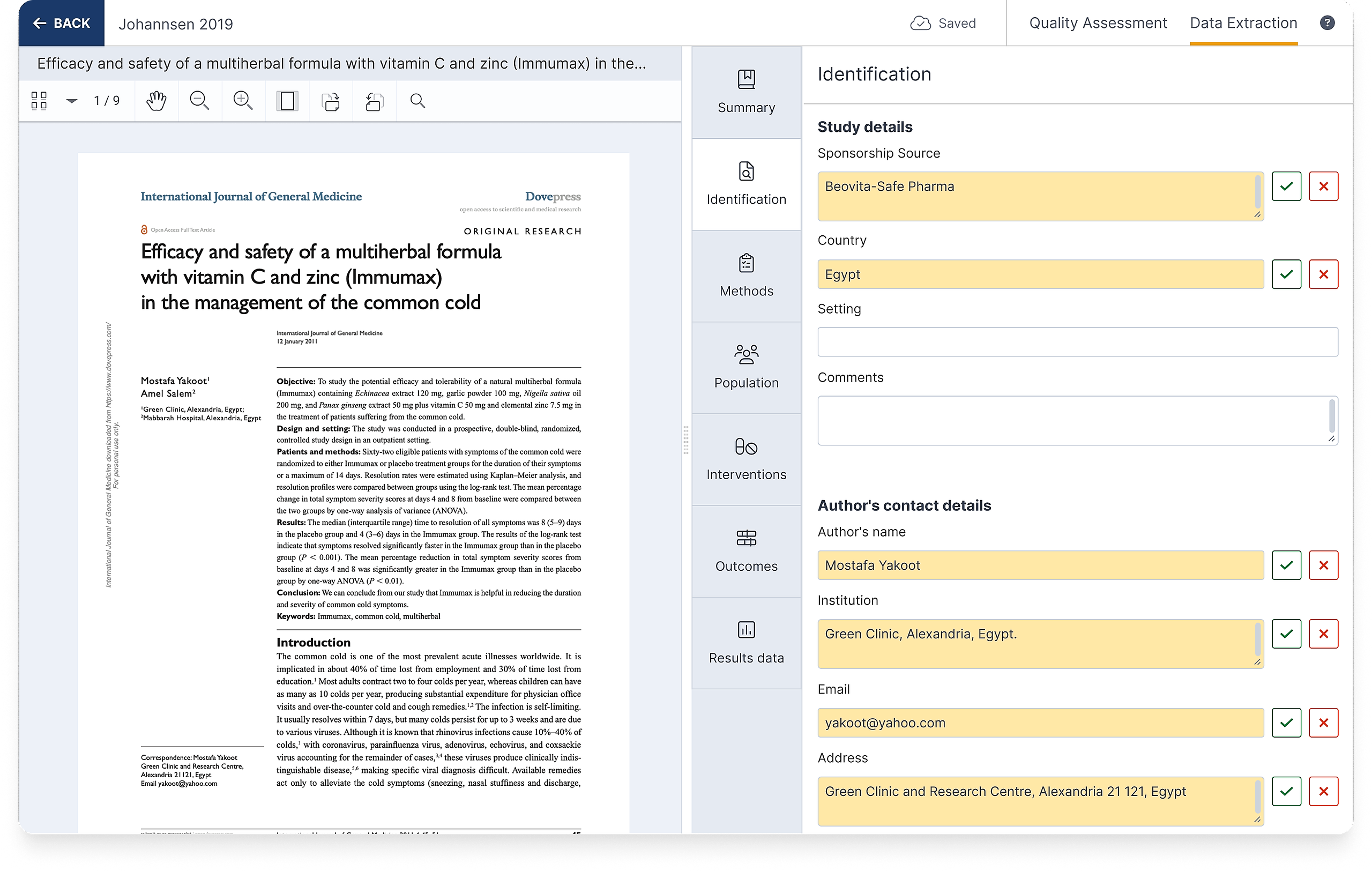
Extracting data from studies
All fields you add to the template will appear on the data extraction form for each study.
Covidence provides automatic extraction in Extraction 1 (DE1) for fields where reliable suggestions are available. A DOI should be available, and these suggestions are enabled by default.
Extraction suggestions will show for all users in DE1, requiring them to either accept or reject each suggestion individually. If we aren’t able to find a suggestion for the field, it will appear as a normal input (no suggestion).
Currently, the data sources include:
• Study metadata from external repositories (for both open and closed access studies)
• Large Language Model (LLM) extraction from full-text articles (or both open and closed access studies)
Help for setting up each section of the template
Below are step by step guides providing tips on how to set up your template for each section:

