Extraction 1 - Data Extraction
How to set up the methods section
Last updated on 22 Oct, 2025
Setting up the template
The methods section of the template is designed to capture details about the design, aims and conduct of the study.
All fields you add to this section will appear for all studies, allowing you to collect consistent data across studies. You can update the template at any time throughout the data extraction process.
Fields within the methods section of the template are listed under two subheadings, described in more detail below.
Method details
There are two default fields under this sub-heading, which can be un-selected in the template. These fields allow you to record key details about the design of the included study:
Design - a dropdown field with seven common designs (e.g. Randomized Control Trial, Case Control Study, Cluster randomised controlled trial, Controlled interrupted time series, Historically controlled trial, Prospective and Retrospective cohort study), and an ‘other’ option to record the design of the study
Group - a multiple choice field to record whether the study had a parallel (two or more groups receiving different interventions at the same time) or crossover design (two or more groups receiving the same interventions in a different order).
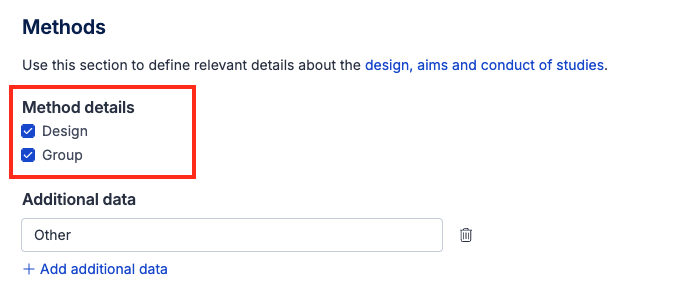
Additional data
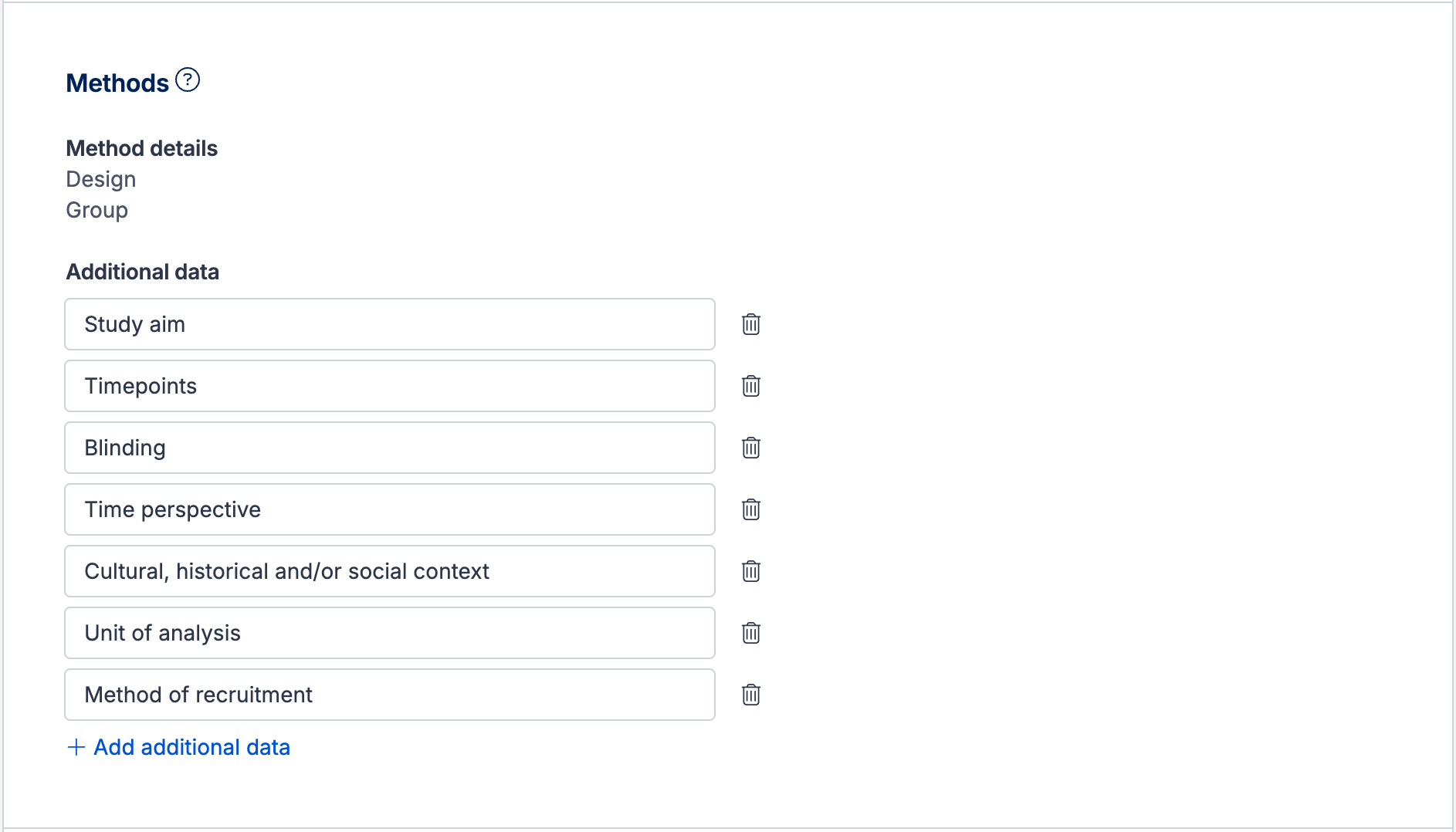
The Additional data subheading includes one default field that you can edit and remove:
Other - A place to extract any other relevant information
Under this subheading you can add custom fields to suit the specifics of your review not captured within the other subheading.
Methods fields added under the additional data subheading will vary depending on what sort of studies are eligible for your review and the specific needs of your review. For example, reviews including non-randomised study designs are likely to need further fields to capture different design features which can be added under this subheading.
Examples of custom fields that may be useful to add under this subheading include:
Study aim - the purpose of the study, for example if some studies were designed to find the optimal dose of a drug and others looked at safety and effectiveness of one dose versus placebo
Timepoints - the main endpoint of the study or the main assessment timepoints for comparison across studies. Note that timepoints can be captured for each result in the Outcomes section.
Blinding - whether there were masking procedures and for whom (participants, investigators, outcome assessors etc)
Time perspective - whether each study had a prospective, retrospective or cross-sectional design, if you plan to include a range of study types
Cultural, historical and/or social context - details about the context in which the study was conducted to consider the applicability of the results
Unit of analysis - participants, centres (e.g. schools or clinics), or parts of the body (e.g. eyes or joints) to consider and correct from at the point of synthesis
Method of recruitment - e.g. via clinics, surveys etc to consider how it may affect the population identified
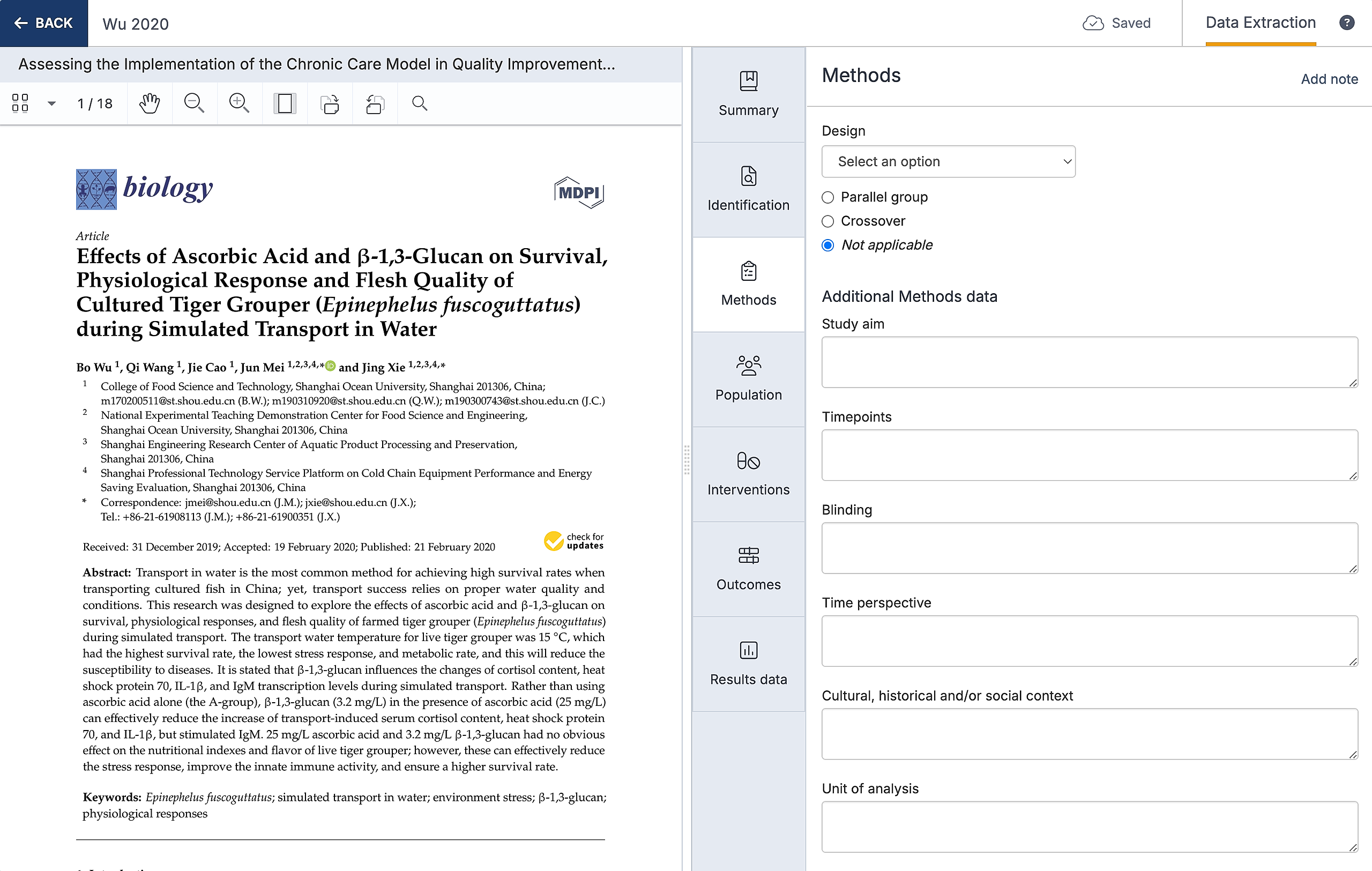
Extracting data from studies
All fields you add to the template will appear on the data extraction form for each study.
Help for setting up each section of the template
Below are step by step guides providing tips on how to set up your template for each section:

